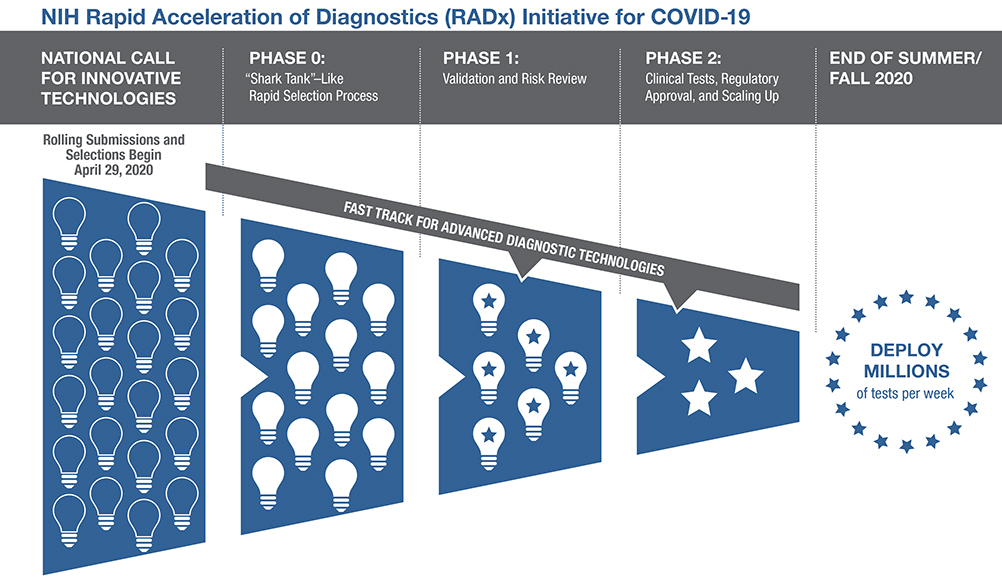
Initiative aims to speed delivery of accurate, easy-to-use, scalable tests to all Americans
The National Institutes of Health announced a new initiative aimed at speeding innovation, development and commercialization of COVID-19 testing technologies, a pivotal component needed to return to normal during this unprecedented global pandemic. With a $1.5 billion investment from federal stimulus funding, the newly launched Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding into early innovative technologies to speed development of rapid and widely accessible COVID-19 testing. At the same time, NIH will seek opportunities to move more advanced diagnostic technologies swiftly through the development pipeline toward commercialization and broad availability. NIH will work closely with the U.S. Food and Drug Administration, the Centers for Disease Control and Prevention and the Biomedical Advanced Research and Development Authority (BARDA) to advance these goals.
More information can be found here.