Four sequential, first-order enzymatic reactions S <--> P with substrates binding to enzymes, and reversible product formation.
Description
Each reaction is an enzymatic conversion of substrate, S, to product, P. After binding solute S to enzyme E to form a substrate-enzyme complex ES, there is a reaction-release event producing the product P and the free enzyme E. The model parameters can be adjusted to fit Michaelis-Menten conditions (Substrate concentrations high compared to enzyme; on/off reaction fast compared to forward reaction rate) and Briggs-Haldane conditions where the On-rate is low.
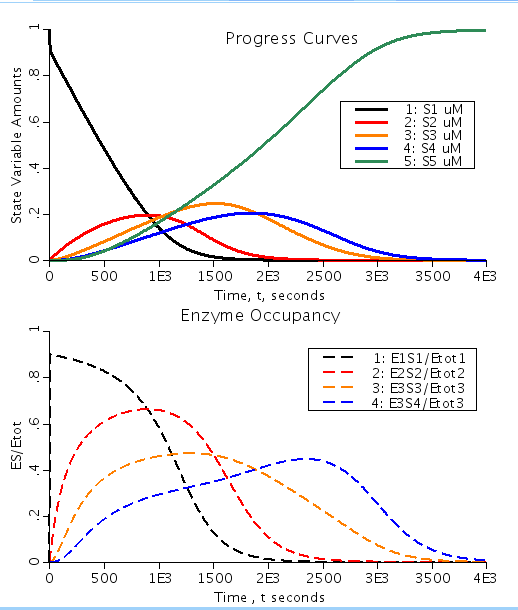
Figure: Shows the rapid equilibration which was assumed by Michaelis and Menten for their reaction kinetic approximation to be valid. This results in the reaction from ES complex to product P being the rate limiting step in the process. Under the conditions set, with Kp >> Kd the reaction is almost irreversible resulting in progressive appearance of the products with almost no effective reversibility. (Kp is the equilibrium dissociation constant for P binding to E and Kd is the equilibrium dissociation constant for substrate S binding to E). Top figure shows the amount of substrate available at each enzymatic reaction step. Bottom figure shows the relative amount of enzyme bound to substrate for each reaction as a function of time.
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
- Download JSim model MML code (text):
- Download translated SBML version of model (if available):
We welcome comments and feedback for this model. Please use the button below to send comments:
Bassingthwaighte JB.: Enzymes and Metabolic Reactions, Chapter 10 in "Transport and Reactions in Biological Systems", Pages 7-8
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

