Multiple tracer dilution estimates of D- and 2-deoxy-D-glucose uptake by the heart using a three region, 7-path, Blood-Tissue-Exchange (BTEX) model for albumin, L-Glucose, D-Glucose, and deoxy-Glucose. From Kuikka J, Levin M, Bassingthwaighte JB 1986 paper.
Reproducible project file example
Download the JSim project file which contains the model source code, data, simulation parameter sets and plot pages needed to reproduce the Kuikka et al. 1986 paper. This is an example of a package that reproduces a model published in a journal.
There are two related models that expand upon this one: Kuikka 1986 experiment, which is the same model as presented here but includes parameter fits to all data summarized in Tables 1 and 2 of Kuikka 1986 paper, and Kuikka 1986 two region BTEX, a simplified, two region model where the permeability and consumption term for the third region (myocardial cells) are replaced with just a consumption term in the second region (interstitial fluid). This model is only fit to data set found in Table 3 of the Kuikka 1986 paper.
MPC: This model was initially created using the Modular Program Constructor (MPC). For more information:
- Detailed MPC information.
- MPC text file that generated model.
- Download zip file containing MPC file and MPC library files used to build this model.
Description
Multiple indicator dilution (MID) analysis for the estimation of capillary permeability in the heart. The MID principle is to analyze the cellular permeability surface area product for the target solute, D-glucose, against two reference solutes, one to characterize the intravascular transport function, href(t) through the intact organ ( (131)I-albumin in this case ), an extracellular one of the same molecular weight as the target solute in order to characterize the combination of permeation through the inter-endothelial cleft (using L-glucose), and the target solute, D-glucose that is taken up by cells and metabolized. KUIKKA, JYRKI, MICHAEL LEVIN, AND JAMES B. BASSINGTHWAIGHTE. Multiple tracer dilution estimates of D- and 2-deoxy-D-glucose uptake by the heart. Am. J. Physiol. 250 (Heart Circ. Physiol. 19): H29-H42, 1986.-Permeability-surface area products of the capillary wall, PSc, and the myocyte sarcolemma, PSpc, for D-glucose and 2-deoxy-D-glucose were estimated via the multiple indicator-dilution technique in isolated blood-perfused dog and Tyrode-perfused rabbit hearts. Aortic bolus injections contained I(131)-albumin (intravascular reference), two of three glucoses: L-glucose (an extracellular reference solute), D-glucose, and 2-deoxy-D-glucose. Outflow dilution curves were sampled for l-2.5 min without recirculation. The long duration sampling allowed accurate evaluation of PS, by fitting the dilution curves with a multiregional axially distributed capillary-interstitial fluid-cell model accounting for the heterogeneity of regional flows (measured using microspheres and total heart sectioning). With average blood flow of 1.3 ml/(g*min), in the dog hearts the PSc, for D-glucose was 0.72 +- 0.17 ml/(g*min) (mean t SD; n = ll), and PSpc, was 0.57 t 0.15 ml/(g*min). In the rabbit hearts with perfusate flow of 2.0 ml/(g*min)(n = 6), PSc, was 1.2 * 0.1 and PSpc, was 0.4 & 0.1 ml/(g*min). PSc, for 2-deoxy-Dglucose was about 4% higher than for D-glucose and L-glucose in both preparations. Relative to L-glucose, there was no measurable transendothelial transport of either dextroglucose, indicating that transcapillary transport was by passive diffusion, presumably via the clefts between cells. The technique allows repeated measurements of D-glucose uptake at intervals of a few minutes; it may therefore be used to assess changes in transport rates occurring over intervals of several minutes.
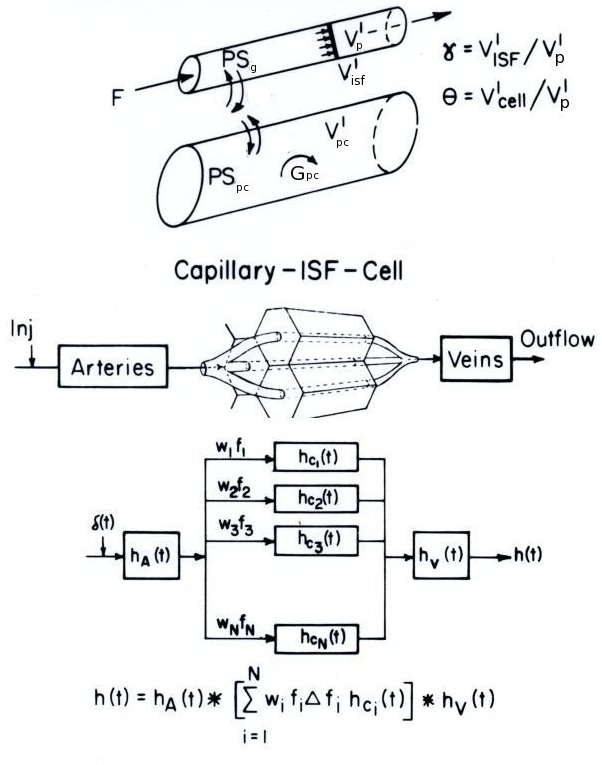
Figure: Model representation of BTEX in the heart. (Top): Single capillary exchange into the ISF and Cellular region (parenchymal cell = pc). (Middle): Multi-path diagram of lung from injector to outlflow collector. (Bottom): Mathematical, linear system representation of the multi-path model.
Kuikka MID Data
The MID glucose datasets published in the Kuikka et al. 1986 paper are available on the Physionet web site. At the Physionet web site search for 'Cardiac Physiome Kuikka glucose' in the PhysioNetWorks area (you will need a free, personal account to access it).
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
- Download JSim model MML code (text):
- Download translated SBML version of model (if available):
- No SBML translation currently available.
- Information on SBML conversion in JSim
We welcome comments and feedback for this model. Please use the button below to send comments:
(Primary) Kuikka J., Levin M., Bassingthwaighte J. B., Multiple tracer dilution estimates of D- and 2-deoxy-D-glucose uptake by the heart, Am. J. Physiol. Heart Circ. Physiol., 250, (H29-H42}, 1986
1. ALVAREZ, 0. A., AND D. L. YUDILEVICH. Heart capillary permeability to lipid-insoluble molecules. J. Physiol. Land. 202: 45-58, 1969. 2. BASSINGTHWAIGHTE, J. B. A concurrent flow model for extraction during transcapillary passage. Circ. Res. 35: 483-503, 1974. 3. BASSINGTHWAIGHTE, J. B., AND C. A. GORESKY. Modeling in the analysis of solute and water exchange in the microvasculature. In: Handbook of Physiology. The Cardiovascular System. Microcirculation. Bethesda, MD: Am. Physiol. Sot., 1984, sect. 2, vol. 4, chapt. 13, p. 549-626. 4. BASSINGTHWAIGHTE, J. B., J. T. KUIKKA, I. S. CHAN, T. ARTS, AND R. S. RENEMAN. A comparison of ascorbate and glucose transport in the heart. Am. J. Physiol. 249 (Heart Circ. Physiol. 18): H141-H149,1985. 5. BASSINGTHWAIGHTE, J. B., A. M. LENHOFF, AND J. L. STEPHENSON. A sliding-element algorithm for rapid solution of spatially distributed convection-permeation models (Abstract). Biophys. J. 45: 175a, 1984. 6. BASSINGTHWAIGHTE, J. B., T. YIPINTSOI, AND R. B. HARVEY. Microvasculature of the dog left ventricular myocardium. Microvast. Res. 7: 229-249, 1974. 7. COUSINEAU, D., C. P. ROSE, D. LAMOUREAUX, AND C. A. Go- RESKY. Changes in cardiac transcapillary exchange with metabolic coronary vasodilation in the intact dog. Circ. Res. 53: 719-730, 1983. 8. CRONE, C. Facilitated transfer of glucose from blood into brain tissue. J. Physiol. Lad. 181: 103-113, 1965. 9. CRONE, C., AND A. M. THOMPSON. Comparative studies of capillary permeability in brain and muscle. Acta Physiol. Stand. 87: 252-260,1973. 10. DURAN, W. N., AND D. L. YUDILEVICH. Estimate of capillary permeability coefficieTrafficnts of canine heart to sodium and glucose. Microvasc. Res. 15: 195-205, 1978. 11. GULLER, B., T. YIPINTSOI, A. L. ORVIS, AND J. B. BASSINGTHWAIGHTE. Myocardial sodium extraction at varied coronary flows in the dog: estimation of capillary permeability by residue and outflow detection. Circ. Res. 37: ‘359-378,1975. 12. HAWKINS, R. A., A. M. MANS, D. W. DAVIS, J. R. VISTA, AND L. S. HIBBARD. Cerebral glucose use measured with [14C]glucose labeled in the 1, 2, or 6 position. Am. J. Physiol. 248 (CeU Physiol. 17): C170-C176,1985. 13. KING, R. B., AND J. B. BASSINGTHWAIGHTE. Radioactivity. In: Data in Medicine: Collection, Processing and Presentation. Instrumentation and Techniques in Clinical Medicine, edited by R. S. Reneman and J. Strackee. The Hague, Netherlands: Nijhoff, 1979, vol. I, p. 79-113. 14. KING, R. B., J. B. BASSINGTHWAIGHTE, J. R. S HALES, AND L. B. ROWELL. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ. Res. 57: 285-295,1985 in a biological sugar transport system. Am. J. Physiol. 194: 333- 337,1958. 16. LENHOFF, A. M., AND E. N. LIGHTFOOT. The effects of axial diffusion and permeability barriers on the transient response of tissue cylinders. II. Solution in time domain. J. Theor. Biol. 106: 207-238,1984. 17. LEVIN, M., J. KUIKKA, AND J. B. BASSINGTHWAIGHTE. Sensitivity analysis in optimization of time-distributed parameters for a coronary circulation model. Med. Prog. Tech& 7: 119-124, 1980. 18. MACCHIA, D. D., E. PAGE, AND P. I. POLIMENI. Interstitial anion distribution in striated muscle determined with [35S]sulfate and [3H]sucrose. Am. J Physiol. 237 (CeZZ Physiol. 6): C125-C130,1979. 19. MORGAN, H. E., D. M. REGEN, AND C. R. PARK. Identification of a mobile carrier-mediated sugar transport system in muscle. J. Biol. Chem. 239: 369-374,1964. 20. OPIE, L. H. Effects of regional ischemia on metabolism of glucose and fatty acids: relative rates of aerobic and anaerobic energy production during myocardial infarction and comparison with effects of anoxia. Circ. Res., Suppl. I, 38: 1-52-I-74, 1976. 21. PARIS, S., J. POUYSSI~GUR, AND G. AILHAUD. Analysis by countertransport, relationship to phosphorylation and effect of glucose starvation. B&him. Biophys. Acta 602: 644-652, 1980. 22. PARK, C. R., D. RINEWEIN, M. J. HENDERSON, E. CADENAS, AND H. E. MORGAN. The action of insulin on the transport of glucose through the cell membrane. Am. J. Med. 26: 674-684,1959. 23. PENPARGKUL, S., J. KUZIAK, AND J. SCHEUER. Effect of uremia upon carbohydrate metabolism in isolated perfused rat heart. J. Mol. Cell. Cardiol. 7: 499-511, 1975. 24. PHELPS, M. E., E. J. HOFFMAN, S. C. HUANG, AND D. E. KUHL. Positron tomography: in vivo autoradiographic approach to measurement of cerebral hemodynamics and metabolism. Acta Neural. Scand, Suppl. 56: 446-447,1977. 25. POLIMENI, P. I. Extracellular space and ionic distribution in rat ventricle. Am. J. Physiol. 227: 676-683, 1974. 26. RAICHLE, M. E., K. B. LARSON, M. E. PHELPS, R. L. GRUBB, JR., M. J. WELCH, AND M. M. TER-POGOSSIAN. In vivo measurement of brain glucose transport and metabolism employing glucose%. Am. J. Physiol. 228: 1936-1948, 1975. 27. RANDLE, P. J., AND P. K. TUBBS. Carbohydrate and fatty acid metabolism. In: Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Sot., 1979, sect. 2, vol. 1, chapt. 23, p. 805-844. 28. ROSE, C. P., AND C. A. GORESKY. Vasomotor control of capillary transit time heterogeneity in the canine coronary circulation. Circ. Res. 39: 541-554, 1976. 29. ROSE, C. P., C. A. GORESKY, AND G. G. BACH. The capillary and sarcolemmal barriers in the heart. An exploration of labeled water permeability. Circ. Res. 41: 515-533, 1977. 30. ROVETTO, M. J., J. T. WHITMER, AND J. R. NEELY. Comparison of the effects of anoxia and whole heart ischemia on carbohydrate utilization in isolated working rat hearts. Circ. Res. 32: 699-711, 1973. 31. SACKS, W., D. C. SCHECHTER, AND S. SACKS. A difference in the in vivo cerebral production of [1-14C]lactate from D-[3-14C]glucose in chronic mental patients. J. Neurosci. Res. 6: 225-236, 1981. 32. SCHAFER, D., AND J. A. JOHNSON. Permeability of mammalian heart capillaries to sucrose and insulin. Am. J. Physiol. 206: 985- 991,1964. 33. SOKOLOFF, L., M. REIVICH, C. KENNEDY, M. H. DES ROSIERS, C. S. PATLAK, K. D. PETTIGREW, 0. SAKURADA, AND M. SHINOHARA. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28: 897- 916,1977. 34. YIPINTSOI, T. Single-passage extraction and permeability estimation of sodium in normal dog lungs. Circ. Res. 34: 523-531, 1976. 35. YIPINTSOI, T., AND J. B. BASSINGTHWAIGHTE. Circulatory transport of iodoantipyrine and water in the isolated dog heart. Circ. Res. 27: 461-477, 1970. 36. YIPINTSOI, T., W. A. DOBBS, P. D. SCANLON, T. J. KNOPP, AND J. B. BASSINGTHWAIGHTE. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ. Res. 33: 573-587, 1973. 37. YIPINTSOI, T., P. D. SCANLON, AND J. B. BASSINGTHWAIGHTE. Density and water content of dog ventricular myocardium. Proc. Sot. Exp. Biol. Med. 141: 1032-1035,1972. 38. YIPINTSOI, T., R. G. TANCREDI, D. R. RICHMOND, AND J. B. BASSINGTHWAIGHTE. Myocardial extractions of sucrose, glucose, and potassium. In: Capillary Permeability. Proceedings of Alfred Benzon Symposium II, edited by C. Crone and N. A. Lassen. Copenhagen: Munksgaard, 1970, p. 153-156. 39. YUDILEVICH, D. L., AND N. DE ROSE. Blood-brain transfer of glucose and other molecules measured by rapid indicator dilution. Am. J. Physiol. 220: 841~846,1971.
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

