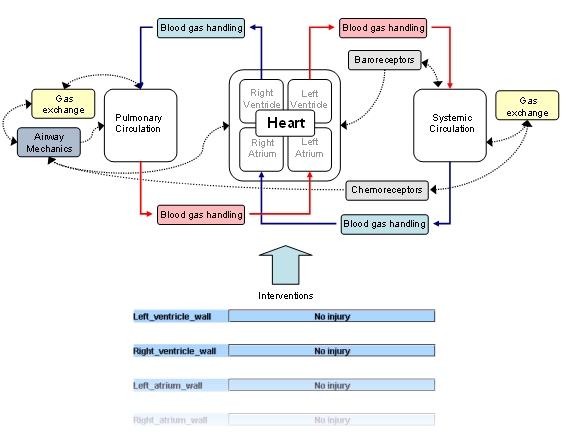
A closed loop cardiopulmonary model composed of a four-chamber varying-elastance heart, a pericardium, a systemic circulation, a pulmonary circulation, airways mechanics, baroreceptors, gas exchange, blood gas handling, coronary circulation, peripheral chemoreceptors and selectable interventions.
Description
The model simulates physiological features such as the arterial blood pressure waveform, end-diastolic left ventricular volume, coronary capillary flow, pleural pressure, and arterial oxygen concentration. The JSim project file also includes a customized interface that allows the user to simulate interventions (injuries, maneuvers, Valsalva maneuver, pericardial removal, penetrating wounds to heart, septal defect, etc.). Interventions not listed in the customized interface can also be simulated by changing certain parameters in the model. For example, the parameter for pulmonary valve resistance (Rpuv) can be increased to simulate pulmonary valve constriction.
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
Validation
Presently, this model has only been partially validated.
- Unstressed steady-state physiology: The cardiovascular system has been tuned to produce blood pressures, blood volumes, blood flows and heart rate within the limits of normal human physiological variation. Running the model after loading the 'unstressed' parameter set will produce these 'textbook' results. Reference values used to tune the model were compiled from various literature sources.
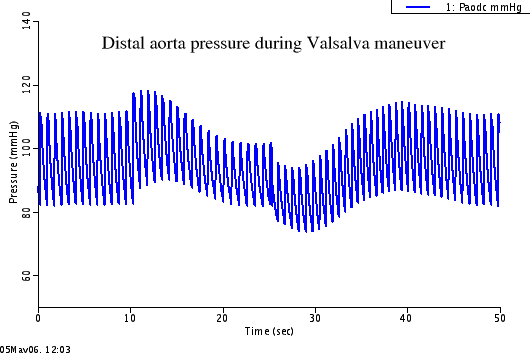
- Valsalva maneuver: The arterial pressure response to a simulated Valsalva maneuver produces the 4 distinct phases associated with a sudden, sustained increase in pleural pressure (sustained, in this case, for 15 seconds) followed by its quick decrease to 'normal' values. Heart rate shifts in accord with a typical response; however, its maximum level during phase 3 is slightly lower than what has been previously used to validate other models (Lu et al. 2001, Bannister 1980).
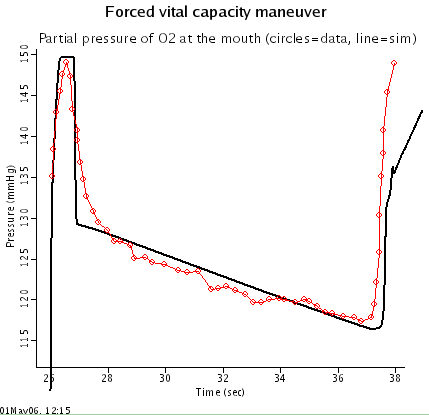
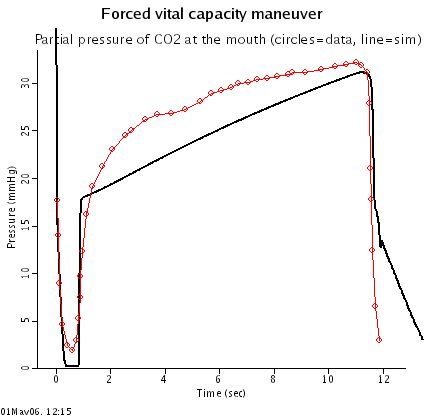
- Forced vital capacity maneuver: To simulate a forced vital capacity (FVC) maneuver, experimental pleural pressure data (Liu et al. 1998) is used to drive the airway mechanics system. The parameters of the airway mechanics system were hand tuned so that the decrease in O2 and increase in CO2 at the mouth during the long expiratory phase of the maneuver matched experimental data (Liu et al. 1998). Although the general trends for O2 and CO2 in this simulation are similar to data, there are differences in the shape of the curves. Further tuning the resistance-volume and pressure-volume relationships in the collapsible and alveolar segments of the airways mechanics model will likely be needed to better match O2 and CO2 trends.
- Removal of pericardium: Removal of the pericardium produces qualitatively valid physiological responses in the model: venous pressure falls, arterial pressure rises, and cardiac output increases (Kuno 1915). These responses appear to be slightly lower in magnitude than what has been found by experiments in dogs (Kuno 1915). To increase these responses, the model must be parameterized so that the pericardium generates more pressure for a given enclosed volume during the normal state (the pressure-volume curve of the pericardial compartment must be shifted to the left).
Notes
- Because the system is highly integrated, numerical solutions for variables in this model converge at very low time steps. The highest time step recommended for a precise solution is 0.001 seconds. A higher time step can be used, and the model will run faster and will use less memory; however, there will be noticeable differences between these results and those produced at lower time steps.
- A reduction in contractile strength is represented by a parameter change. One way to do this here is to use the structural injury choices, for example the decrease in cardiac elastance due to the severance of coronary perfusion, e.g. percent lost set to 95% , starting at 4 seconds and running the total time at 30 seconds.
Observe the changes in Elv, Vlvr or EmaxlvINJ and the overall circulatory responses on plotpage for heart chamber pressures. - Assumptions and limitations.
- Differences betweeen this model and its literature sources.
- This model is informally refered to as VS001 since it was initially created as part of The Virtual Soldier Project.
- Components of this system are based on models already published in the literature. Various people coded these components into MML so they could be integrated together:
CODE COMPONENT CODED BY BASED ON ---------------------------------------------------------------- systemic circulation Dan Beard Lu et al. (2001) pulmonary circulation Dan Beard Lu et al. (2001) baroreceptor Dan Beard Lu et al. (2001) heart Jim Bassingthwaighte Rideout (1991) cardiac activation function Andy Matuszkiewicz Heldt et al. (2002) pericardium Maxwell Neal Sun et al. (1997) airways mechanics Melissa Krueger Athanasiades et al. (2000) gas exchange Kalyan Vinnakota Lu et al. (2001) physiological changes Maxwell Neal Poiseiulle, Reynold's laws blood gas handling Ranjan K. Dash Woodbury (1974) peripheral chemoreceptors Maxwell Neal Lu et al. (2002) Duffin et al. (2000) coronary circulation Maxwell Neal Zinemanas et al. (1994) - This model was published in
- Download JSim model MML code (text):
- Download translated SBML version of model (if available):
We welcome comments and feedback for this model. Please use the button below to send comments:
Athanasiades A, Ghorbel F, Clark JW, Niranjan SC, Olansen J, Zwischenberger JB, Bidani A. Energy analysis of a nonlinear model of the normal human lung. Journal of Biological Systems. 8(2): 115-139, 2000. Brandenburg, Robert O. Cardiology: fundamentals and practice. Yearbook medical publishers, 1987. p. 49. Chung DC, Niranjan SC, Clark Jr. JW, Bidani A, Johnston WE, Zwischenberger JB, Traber DL. A dynamic model of ventricular interaction and pericardial influence. Am J Phsiol Heart Circ Physiol. 272: H2942-H2962, 1997. Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respiration Physiology 120:13-26, 2000. Golden JF, Clark JW, Stevens PM. Mathematical Modeling of Pulmonary Airway Dynamics. IEEE Transactions on Biomedical Engineering. 20(6): 397-404, 1973. Heldt T, Shim EB, Kamm RD, Mark RG. Computational modeling of cardiovascular response to orthostatic stress. Journal of Applied Physiology. 92: 1239-1254, 2002. Kerckhoffs RCP, Neal ML, Gu Q, Bassingthwaighte JB, Omens JH, and McCulloch AD; Coupling of a 3D finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonary circulation. Ann Biomed Eng 35: 1-18, 2007. Kezdi P, and Geller E. Transfer characteristics of the carotid sinus pressure control system. In: Baroreceptors and Hypertension, Kezdi, P. (ed.) Pergamon, Dayton, OH, 1967, pp. 31-40. Liu CH, Niranjan SC, Clark JW, San KY, Zwischenberger JB, Bidani A. Airway mechanics, gas exchange, and blood flow in a nonlinear model of the normal human lung. Journal of Applied Physiology. 84(4): 1447-1469. Lu K, Clark JW, Ghorbel FH, Ware DL, Bidani A. A human cardiopulmonary system model applied to the analysis of the Valsalva maneuver. Am J Physiol Heart Circ Physiol. 281: H2661-H2679, 2001. Lu K, Clark JW, Ghorbel FH, Ware DL, Zwischenberger JB, Bidani A. Whole-body gas exchange in human predicted by a cardiopulmonary model. Cardiovascular Engineering: An International Journal. 3(1):1-19, 2002. Neal ML and Bassingthwaighte JB; Subject-specific model estimation of cardiac output and blood volume during hemorrhage. Cardiovasc Eng 7: 97-120, 2007 Rideout VC. Mathematical computer modeling of physiological systems. Englewood Cliffs, NJ: Prentice Hall, 1991, 261 pp. Sun Y, Beshara M, Lucariello RJ, Chiaramida SA. A comprehensive model for right-left heart interaction under the influence of pericardium and baroreflex. Am J Physiol Heart Circ Physiol. 272: H1499-H1515, 1997. Woodbury, JW. Body acid-base and its regulation. In: Physiology and Biophysics Volume II: Circulation, Respiration, and Fluid Balance. Ruch, TC and Patton, HD (eds.) W.B. Saunders Company. 1974. 558 pp. Zinemanas D, Beyar R, Sideman S. Relating mechanics, blood flow and mass transport in the cardiac muscle. Int. J. Heat Mass Transfer. 37(suppl. 1) 191-205, 1994.
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

