A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation.
Description
A mathematical model of calcium dynamics in vascular smooth muscle cell (SMC) was developed based on data mostly from rat mesenteric arterioles. The model focuses on (a) the plasma membrane electrophysiology; (b) Ca2+ uptake and release from the sarcoplasmic reticulum (SR); (c) cytosolic balance of Ca2+, Na+, K+, and Cl- ions; and (d) IP3 and cGMP formation in response to norepinephrine (NE) and nitric oxide (NO) stimulation. Stimulation with NE induced membrane depolarization and an intracellular Ca2+ ([Ca2+]i) transient followed by a plateau. The plateau concentrations were mostly determined by the activation of voltage-operated Ca2+ channels. NE causes a greater increase in [Ca2+]i than stimulation with KCl to equivalent depolarization. Model simulations suggest that the effect of [Na+]i accumulation on the Na+/Ca2+ exchanger (NCX) can potentially account for this difference. Elevation of [Ca2+]i within a concentration window (150-300 nM) by NE or KCl initiated [Ca2+]i oscillations with a concentration-dependent period. The oscillations were generated by the nonlinear dynamics of Ca2+ release and refilling in the SR. NO repolarized the NE-stimulated SMC and restored low [Ca2+]i mainly through its effect on Ca2+ activated K+ channels. Under certain conditions, (Na+)-(K+)-(ATPase) inhibition can result in the elevation of [Na+]i and the reversal of NCX, increasing resting cytosolic and SR Ca2+ content, as well as reactivity to NE. Blockade of the NCX's reverse mode could eliminate these effects. We conclude that the integration of the selected cellular components yields a mathematical model that reproduces, satisfactorily, some of the established features of SMC physiology. Simulations suggest a potential role of intracellular Na+ in modulating Ca2+ dynamics and provide insights into the mechanisms of SMC constriction, relaxation, and the phenomenon of vasomotion. The model will provide the basis for the development of multi-cellular mathematical models that will investigate microcirculatory function in health and disease. J Theor Biol. 2008 Jul 21;253(2):238-60 This code reproduces Fig. 6 showing effect of nitric oxide on Ca_i and V_m. To enable agonist-induced oscillations, set k_leak = 5, otherwise k_leak = 1. To remove desensitization to NE, set k_pG = 0. Adam Kapela a, Anastasios Bezerianos b, Nikolaos M. Tsoukias a, a Department of Biomedical Engineering, Florida International University, Miami, FL 33199, USA b Department of Medical Physics, University of Patras, Patras, Greece
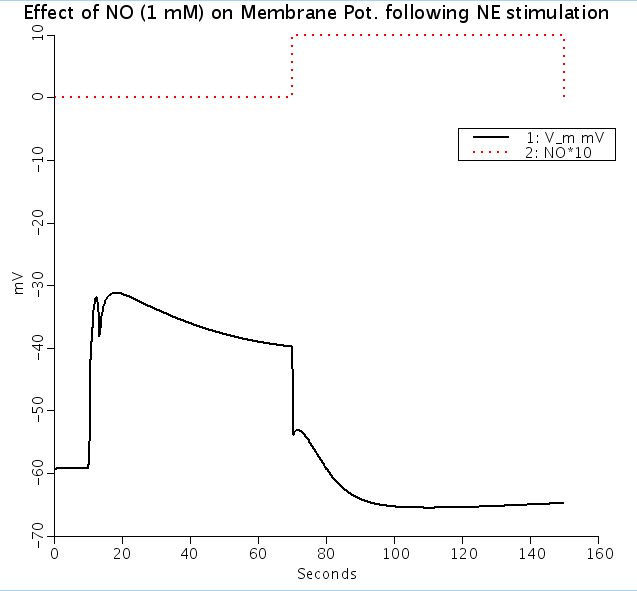
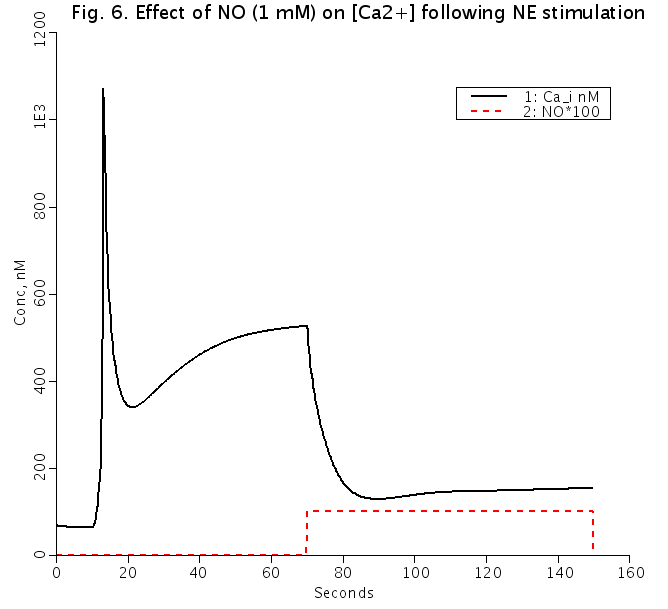
Figures: Effect of 1 mM nitric oxide(NO) on Vm (top) and [Ca2+]i (bottom) following NE stimulation (solid lines). Dashed red lines represent time period of NO addition and are shown for timing purposes only.
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
We welcome comments and feedback for this model. Please use the button below to send comments:
Kapela A, Bezerianos A, Tsoukias NM.: A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation, J Theor Biol 253:238-260, 2008 Silva HS, Kapela A, Tsoukias NM: A mathematical model of plasma membrane electrophysiology and calcium dynamics in vascular endothelial cells. Am J Physiol Cell Physiol 293:C277-C293, 2007 Kapela A, Bezerianos A, Tsoukias NM: A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication, MICROCIRC 16:8,(694-U69), 2009
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

