4 Region Axially Distributed Multi Path Michaelis-Menten Model applied to analysis of serotonin uptake by lung tissue following injection into pulmonary artery.
Description
4 Region Axially Distributed Model applied to analysis of serotonin
uptake by lung tissue following injection into pulmonary artery.
from article:
- "The Uptake and Metabolism of Substrate...".by Linehan et al
- In "Whole Organ Approaches to Cellular Metabolism", chp 17
- Edited by Bassingthwaighte, Goresky, Linehan
- Look at serotonin (5-HT) specifically.
Uptake into the endothelial and parenchymal regions are modeled using a
Michaelis-Menten transporter which is two-sided, concentration dependent.
Three curve model used to fit physiological variables to three
sets of data simulataneously.
- each separate curve has aaa, bbb, ccc, suffix on the model variable.
- Example: CMpaaa(t,x): Conc curve one for mother in plasma.
- CMpbbb(t,x): Conc curve two "
- CMpccc(t,x): Conc curve three "
- PSg: Passive conductance channel between Plasma and ISF
- PSecl: COncentration dependent transporter between Plasma and EC
- PSeca: Concentration dependent trans between ISF and EC
- PSpc: Conc dependent transporter between ISF and PC
- Gec: EC consumption, can be set to zero.
- Gpc: PC consumption
Multiple Indicator Dilution model.
4 compartment model that includes a vascular reference and
serotonin tracer curves.
Assumptions:
- Tracer (14C-5-HT) << then Mother (5-HT)
- Competition between Mother and Tracer across membranes.
- No counter transport of 5-HT used in calculations for PSecl, PSeca, and PSpc.
- Vascular reference stays within capillary.
- To shorten computation time no ISF reference is used.
Sucrose can be used as a ISF reference.
- Sucrose and Serotonin PSg are equal.
Capillary heterogeneity is taken into account.
- This is done by creating separate paths and assigning a relative mass to each,
- creating a simple probabilty density function based on flow (Fp).
- Currently, seven (7) paths used.
- Example paths for first curve (CMpaaa(t,x)): CMpaa1(t,x), CMpab1(t,x), CMpac1(t,x)
- Path flows are relative to average plasma flow.
Constant infusion:
- Model can accomadate constant infusion of Mother substrate into system.
- At time t.min the arteriol has a concentration of CMpaaa_init.
- WHen a bolus is injected then at x1.min = Cin + CMpaaa_init.
- Can have three different infusion rates, one for each curve.
- Assumption: THere is no uptake of Mother in Arteriols.
- Note: if just want whole system at a const Mother(5-HT) conc then modify the BCs
- so that: when (t=t.min) { CMvaa1 = 0; } becomes:
- when (t=t.min) { CMvaa1 = CMpaaa_init; }, etc...
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
The Blood tissue exchange model and equations:
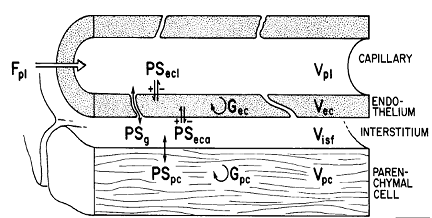
Figure 1. Diagram of a four region blood tissue exchange (Bassingthwaighte 1989).Dec, Disf, and Dpc are zero. Gp and Gisf are zero.
Figure 1 shows a diagram of the four region model. The four regions are described by the following four equations:
Capillary:
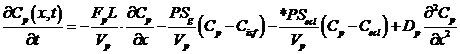 ,
,
Endothelium:
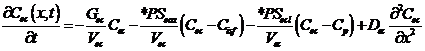 ,
,
Interstitial:
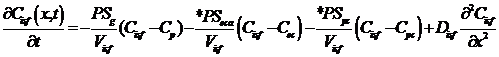 , and
, and
Parenchymal: 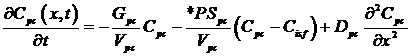
where Cp, Cec, Cisf, and Cpc are the concentrations (mol/ml) of the substrate in the capillary, endothelial cell (EC), interstitial fluid space (ISF), and parenchymal cell (PC) respectively, dependent on axial position x and time t. *PS indicate saturable transporters. Fp is plasma flow in ml/(g*min), Vp, Vec, Visf, and Vpc are volumes of distribution (ml/g) within the plasma, ec, isf, and pc respectively (in this paper V and V' both represent volumes of distribution for the substrate in question). Volumes of distribution are anatomical volumes for a substrate and are concentration dependent. For passive exchanges between regions the volumes of distribution are directly related to the ratio of concentrations between regions. Where steady-state fluxes exist between regions, the volumes are based on concentrations of the substrate and substrate complex at equilibrium. Dp , Dec, Disf, Dpc are the effective axial diffusion coefficients (cm2/sec). Gec and Gpc are consumption terms in ml/(g*min) for the EC and PC. PSg is the diffusive permeability-surface area term used to describe the rate of diffusion between the plasma and the ISF. The permeability-surface area products (PS) PSecl, PSeca, and PSpc describe saturable transporters in ml/(g*min) for substrate transport between plasma-EC, EC-ISF, and ISF-PC respectively.
Endothelial Serotonin transporters:
Endothelial uptake of serotonin can be modeled as a facilitated saturable transporter that in normal physiological states obeys Michaelis-Menten kinetics. This is based on previous multiple indicator dilution (MID) experiments that have shown serotonin concentrations that can saturate the transporter (Rickaby 1981 1982; Peeters 1989; Malcorps 1984). The serotonin transporter is modeled using Michaelis-Menten kinetic parameters Vmax �and Michaelis constant Km:
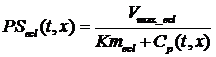 ,
,
where Cp is the total concentration of serotonin in the capillary plasma and the flux through the transporter from capillary to EC is equal to:
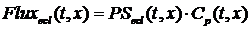
Since the labeled serotonin (tracer) concentration is assumed insignificant compared to the unlabeled serotonin (mother) concentration then CMp can replace Cp:
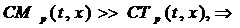

PSecl and PSeca are assumed to be identical in kinetic behavior and Vmax_ecl and Km are the same for each. The use of a simplified transporter is based on two assumptions: the serotonin binding and unbinding reactions with the membrane transporter are fast compared to the movement of the serotonin-transporter complex from one side to the other side of the membrane itself and the transporter concentration is small compared to the substrate serotonin. Based on the first assumption, the transporter is considered a non-capacitance transporter.
Capillary flow heterogeneity:
The modeling of capillary flow heterogeneity is done using a probability density function (PDF) of relative regional flows, w(f). This is further simplified by using a number of discrete flows, instead of a continuous range, to represent the capillary heterogeneity of the lung. Since we are modeling relative flows, f, to the mean capillary flow rate, by definition, w(f) has a mean of 1 and its area is one (King 1996). With seven capillaries being used this simplifies, for n=7, to:
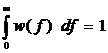 , reduces to:
, reduces to: 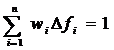
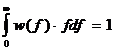 , reduces to:
, reduces to: 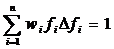
where wi is the frequency of occurrence, within a mass fraction of the organ, in the range Δfi about relative flow fi . The PDF used has a relative dispersion of 0.5 based on reported results for the capillary dispersion within the lung (Knopp et al, 1969). A random walk function is used to represent the PDF with a RD of 0.5 and skewness of 1.6. The relative flow range used varied by dataset but was usually between 0.5 and 2.0.
Arteriole and venule heterogeneity are approximated using the same technique. The arterioles and venules are treated as �pipes� in that there is no uptake or metabolism of serotonin. They only add dispersion and a time delay between input curve and output curve.
- Download JSim model MML code (text):
- Download translated SBML version of model (if available):
- No SBML translation currently available.
- Information on SBML conversion in JSim
We welcome comments and feedback for this model. Please use the button below to send comments:
- Bassingthwaighte, J.B., C.Y. Wang, and I.S. Chan: "Blood-Tissue Exchange Via Transport
and Transformation by Capillary Endothelial-Cells". Circulation Research, 1989. 65(4): p. 997-1020.
- Bronikowski, T.A., et al.: "A Mathematical-Model of Indicator Extraction by the Pulmonary
Endothelium Via Saturation Kinetics". Mathematical Biosciences, 1982. 61(2): p. 237-266.
- Dawson, C.A., et al.: "Kinetics of Serotonin Uptake in the Intact Lung".
Annals of Biomedical Engineering, 1987. 15: p. 217-227.
- King, R.B., G.M. Raymond, and J.B. Bassingthwaighte: "Modeling blood flow heterogeneity".
Annals of Biomedical Engineering, 1996. 24(3): p. 352-372.
- Linehan, J.H., S.H. Audi, and C.A. Dawson: "The Uptake and Metabolism of Substrates in
the Lung" in Whole Organ Approaches to Cellular Mechanism, J. Bassingthwaighte, C.A. Goresky,
and J.H. Linehan, Editors. 1998, Springer-Verlag: New York. p. 427-437.
- Linehan, J.H., T.A. Bronikowski, and C.A. Dawson: "Kinetics of Uptake and Metabolism by
Endothelial-Cell from Indicator Dilution Data". Annals of Biomedical Engineering, 1986. 14(1): p. 87-87.
- Malcorps, C.M., et al." "Lung Serotonin Uptake Kinetics from Indicator-Dilution and
Constant-Infusion Methods". Journal of Applied Physiology, 1984. 57(3): p. 720-730.
- Rickaby, D.A., C.A. Dawson, and J.H. Linehan: "Influence of Blood and Plasma-Flow Rate
on Kinetics of Serotonin Uptake by Lungs". Journal of Applied Physiology, 1982. 53(3): p. 677-684.
- Rickaby, D.A., et al.: "Kinetics of Serotonin uptake in the dog lung". J. Appl. Physiol,
1981. 51: p. 405-414.
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

