This model analyzes the influence of voltage-dependent calcium (Ca2+)-independent transient current (Ito1) on the action potential duration (APD) in normal vs failing canine and human cardiac myocytes.
Description
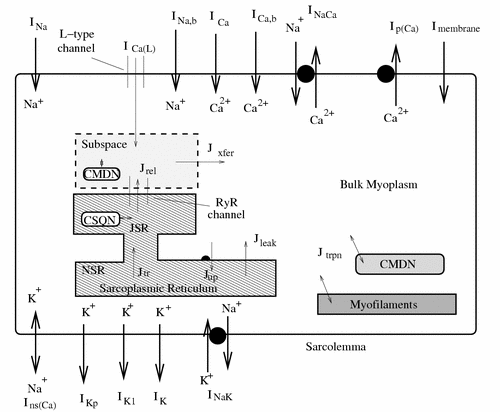
Ca2+ transients measured in failing human ventricular myocytes exhibit reduced amplitude, slowed relaxation, and blunted frequency dependence. In the companion article (O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marbán E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart, I: experimental studies. Circ Res. 1999;84:562-570), O'Rourke et al show that Ca2+ transients recorded in myocytes isolated from canine hearts subjected to the tachycardia pacing protocol exhibit similar responses. Analyses of protein levels in these failing hearts reveal that both SR Ca2+ ATPase and phospholamban are decreased on average by 28% and that Na+/Ca2+ exchanger (NCX) protein is increased on average by 104%. In this article, we present a model of the canine midmyocardial ventricular action potential and Ca2+ transient. The model is used to estimate the degree of functional upregulation and downregulation of NCX and SR Ca2+ ATPase in heart failure using data obtained from 2 different experimental protocols.
Equations
The equations for this model may be viewed by running the JSim model applet and clicking on the Source tab at the bottom left of JSim's Run Time graphical user interface. The equations are written in JSim's Mathematical Modeling Language (MML). See the Introduction to MML and the MML Reference Manual. Additional documentation for MML can be found by using the search option at the Physiome home page.
- Download JSim model MML code (text):
- Download translated SBML version of model (if available):
We welcome comments and feedback for this model. Please use the button below to send comments:
Winslow R.L., Rice J., Jafri S., Marban E., and O'Rourke B., Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, II: model studies. Circ Res. 1999 Mar 19;84(5):571-86.
Greenstein J.L., Wu R., Po S., Tomaselli G.F., and Winslow, R.L., Role of the Calcium-independent transient outward current Ito1 in shaping action potential morphology and duration. Circ Res, 1026-1033, 2000.
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

