(Matlab) Catalytic Coupling of Oxidative Phosphorylation, ATP Demand, and Reactive Oxygen Species Generation.
Description
(Abstract) Competing models of mitochondrial energy metabolism in the heart are highly disputed. In addition, the mechanisms of reactive oxygen species (ROS) production and scavenging are not well understood. To deepen our understanding of these processes, a computer model was developed to integrate the biophysical processes of oxidative phosphorylation and ROS generation. The model was calibrated with experimental data obtained from isolated rat heart mitochondria subjected to physiological conditions and workloads. Model simulations show that changes in the quinone pool redox state are responsible for the apparent inorganic phosphate activation of complex III. Model simulations predict that complex III is responsible for more ROS production during physiological working conditions relative to complex I. However, this relationship is reversed under pathological conditions. Finally, model analysis reveals how a highly reduced quinone pool caused by elevated levels of succinate is likely responsible for the burst of ROS seen during reperfusion after ischemia.
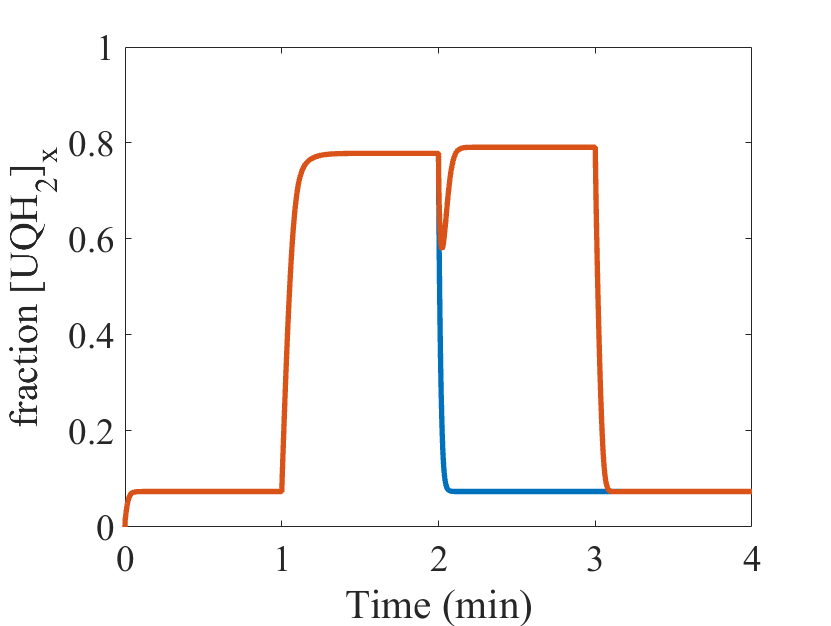
Figure 1: (From Bazil et al. 2016 Fig 6) Model simulations of ischemia/reperfusion (I/R). Two I/R simulations are run for the I/R protocol timeline shown (blue, orange). The model parameters are given in Table 1 and the Supporting Material. For the initialization period, the O2 concentration is set to 30 mM, and the ATPase activity is set to 2 mM/s to produce a moderate workload demand. For the ischemic period, the O2 concentration is set to 1 nM to reflect the hypoxic conditions during ischemia. For the reperfusion period, the O2 concentration is reset to its original value for both I/R Simulations 1 and 2. For I/R Simulation 2, the complex II activity and its apparent equilibrium constant are both increased by a factor of 10 to account for an accumulation of succinate that occurs during ischemia. In the recovery period, the model parameters are reset to the conditions for the initialization period. The steady-state model output for fraction of UQH2, ROS production and O2 consumption are shown for Fig1 – Fig3, respectively.
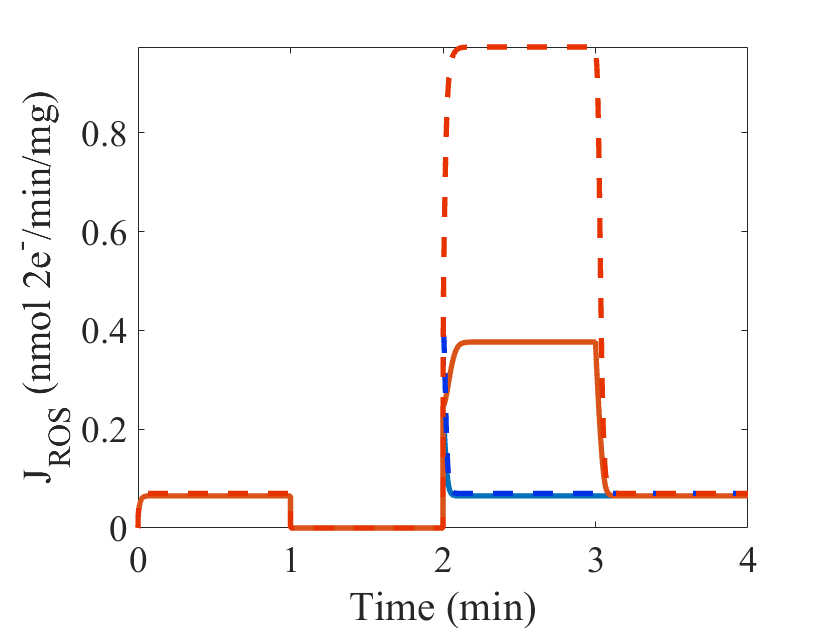
Figure 2: The steady-state model output for ROS production (JROS). For the I/R Simulation 2 conditions, reverse electron transport (RET) is the dominant source of ROS and leads to a 10-fold higher ROS production rate (red dashed) compared to forward electron transport (as compared to ROS production during the initialization period). This is only possible when there is a buildup of substrates that directly lead to UQH2 (e.g., succinate).
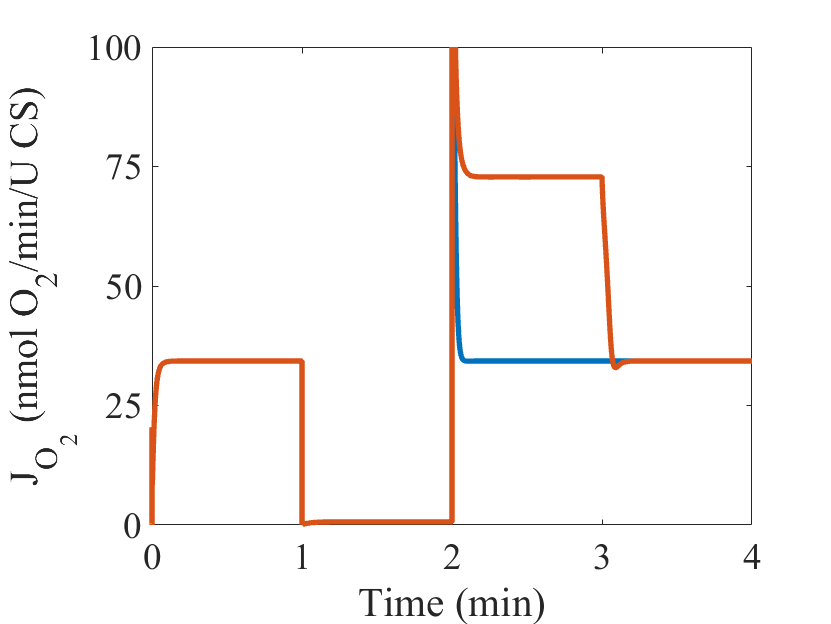
Figure 3: The steady-state model output for O2 consumption (JO2).
Equations
The equations for this model may be viewed by opening up the Matlab model files downloaded from below.
Download Matlab model and associated files.
We welcome comments and feedback for this model. Please use the button below to send comments:
- (Primary) Jason N. Bazil, Daniel A. Beard, Kalyan C. Vinnakota, Catalytic Coupling of Oxidative Phosphorylation, ATP Demand, and Reactive Oxygen Species Generation, Biophysical Journal, Volume 110, Issue 4, 2016, Pages 962-971, ISSN 0006-3495, https://doi.org/10.1016/j.bpj.2015.09.036.
- F. Wu, E.Y. Zhang, et al., D.A. Beard, Phosphate metabolite concentrations and ATP hydrolysis potential in normal and ischaemic hearts, J. Physiol., 586 (2008), pp. 4193-4208
- K.C. Vinnakota, R.K. Dash, D.A. Beard, Stimulatory effects of calcium on respiration and NAD(P)H synthesis in intact rat heart mitochondria utilizing physiological substrates cannot explain respiratory control in vivo, J. Biol. Chem., 286 (2011), pp. 30816-30822
- S. Bose, S. French, et al., R.S. Balaban, Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate J. Biol. Chem., 278 (2003), pp. 39155-39165
Please cite https://www.imagwiki.nibib.nih.gov/physiome in any publication for which this software is used and send one reprint to the address given below:
The National Simulation Resource, Director J. B. Bassingthwaighte, Department of Bioengineering, University of Washington, Seattle WA 98195-5061.
Model development and archiving support at https://www.imagwiki.nibib.nih.gov/physiome provided by the following grants: NIH U01HL122199 Analyzing the Cardiac Power Grid, 09/15/2015 - 05/31/2020, NIH/NIBIB BE08407 Software Integration, JSim and SBW 6/1/09-5/31/13; NIH/NHLBI T15 HL88516-01 Modeling for Heart, Lung and Blood: From Cell to Organ, 4/1/07-3/31/11; NSF BES-0506477 Adaptive Multi-Scale Model Simulation, 8/15/05-7/31/08; NIH/NHLBI R01 HL073598 Core 3: 3D Imaging and Computer Modeling of the Respiratory Tract, 9/1/04-8/31/09; as well as prior support from NIH/NCRR P41 RR01243 Simulation Resource in Circulatory Mass Transport and Exchange, 12/1/1980-11/30/01 and NIH/NIBIB R01 EB001973 JSim: A Simulation Analysis Platform, 3/1/02-2/28/07.

